Our main products: Amino silicone, block silicone, hydrophilic silicone,all of their silicone emulsion,wetting rubbing fastness improver, water repellent(Fluorine free,Carbon 6,Carbon 8), demin washing chemicals(ABS, Enzyme, Spandex protector, Manganese remover),more detail please contact : Mandy +86 19856618619 (Whatsapp )
Introduction to Surfactants
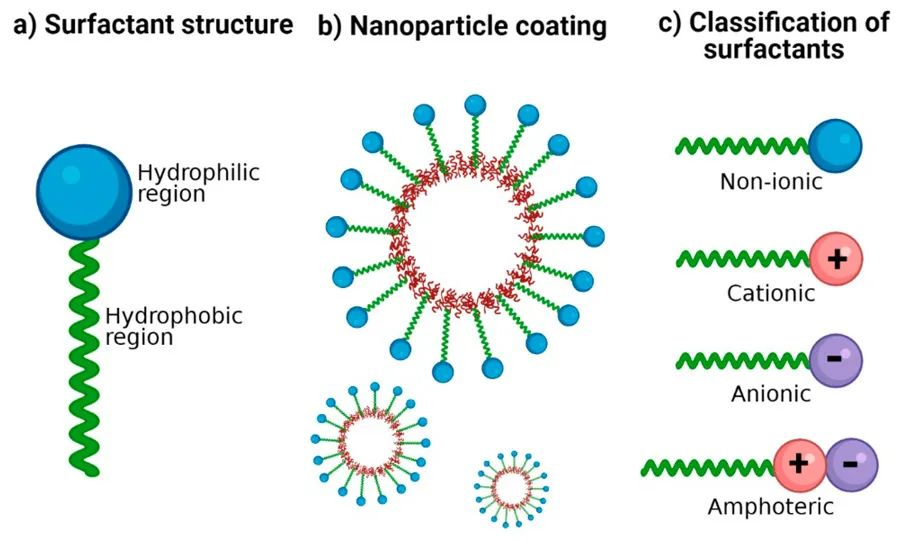
Surfactants possess an amphiphilic molecular structure: one end contains a hydrophilic group, referred to as the hydrophilic head, while the other end contains a hydrophobic group, known as the hydrophobic tail. The hydrophilic head allows surfactants to dissolve in water in their monomer form.
The hydrophilic group is often a polar group, which can be a carboxyl group (-COOH), a sulfonic acid group (-SO3H), an amino group (-NH2), amines and their salts, hydroxyl groups (-OH), amide groups, or ether linkages (-O-) as other examples of polar hydrophilic groups.
The hydrophobic group is typically a non-polar hydrocarbon chain, such as hydrophobic alkyl chains (R- for alkyl) or aromatic groups (Ar- for aryl).
Surfactants can be categorized into ionic surfactants (including cationic and anionic surfactants), non-ionic surfactants, amphoteric surfactants, mixed surfactants, and others. In surfactant solutions, when the concentration of the surfactant reaches a certain value, surfactant molecules will form various ordered aggregates known as micelles. The process of micellization, or micelle formation, is a crucial fundamental property of surfactant solutions, as many important interfacial phenomena are associated with the formation of micelles.
The concentration at which surfactants form micelles in solution is referred to as the Critical Micelle Concentration (CMC). Micelles are not fixed, spherical structures; rather, they exhibit extreme irregularity and dynamic shape changes. Under certain conditions, surfactants may also exhibit reverse micelle states.
Factors Influencing CMC:
- Structure of the surfactant
- Type and presence of additives
- Temperature
Interactions Between Surfactants and Proteins
Proteins contain non-polar, polar, and charged groups, and many amphiphilic molecules can interact with proteins in various ways. Depending on conditions, surfactants can form molecular organized aggregates with different structures, such as micelles or reverse micelles, which interact differently with proteins.
The interactions between proteins and surfactants (Protein-Surfactant, P-S) primarily involve electrostatic interactions and hydrophobic interactions. Ionic surfactants interact with proteins mainly through the electrostatic forces of the polar group and the hydrophobic interactions of the aliphatic carbon chain, binding to the polar and hydrophobic regions of the protein, thus forming P-S complexes.
Non-ionic surfactants primarily interact with proteins through hydrophobic forces, where the hydrophobic chains interact with the hydrophobic regions of the proteins. The interaction can influence both the structure and function of the surfactant and the protein. Therefore, the type and concentration of surfactants, along with the environmental context, determine whether surfactants stabilize or destabilize proteins, as well as whether they promote aggregation or dispersion.
HLB Value of Surfactants
For a surfactant to exhibit its unique interfacial activity, it must balance the hydrophobic and hydrophilic components. The HLB (Hydrophile-Lipophile Balance) is a measure of the hydrophilic-lipophilic balance of surfactants and serves as an indicator of the surfactants' hydrophilic and hydrophobic properties.
The HLB value is a relative value (ranging from 0 to 40). For instance, paraffin has an HLB value of 0 (no hydrophilic component), polyethylene glycol has an HLB value of 20, and the highly hydrophilic SDS (sodium dodecyl sulfate) has an HLB value of 40. The HLB value can serve as a guiding reference when selecting surfactants. A higher HLB value indicates better hydrophilicity, while a lower HLB value suggests poorer hydrophilicity.
Post time: Sep-10-2024

